
Receive Free Shipping On All Systems + Orders
Water Temperature doesn't affect your pH! …Or Does It?
Believe it or not, water pH value is directly related to its temperature. This is due to changes happening to the water on a molecular level at given temperatures. A rise in water temperature leads to molecular vibrations allowing the water to ionize resulting in the formation of more hydrogen ions. When there are more hydrogen ions in your water solution, the pH will fall. In the same regard, if water temperature is lowered, the pH will rise.
In a previous blog post, we talked about the importance of pH and understanding it in hydroponics. Give that post a quick read to fully understand the rest of this blog!
(https://www.hydraunlimited.com/blogs/hydra-blog/understanding-ph-and-ppm-in-hydroponics)
How much is the pH value affected?
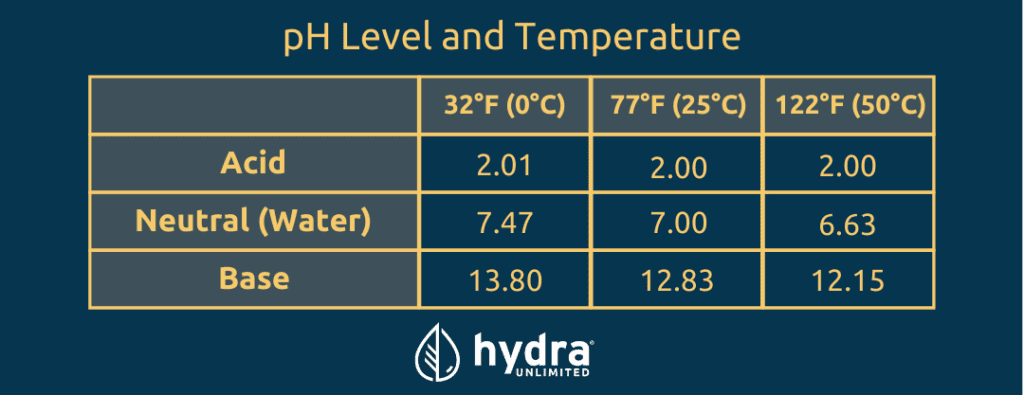
We all know the optimal pH for cannabis growth in hydroponics is important and there isn't much room for error, otherwise your plants may perish. Viewing the table below, we can deduce that variations in temperature of acidic, neutral, and basic solutions all result in pH changes as the temperature lowers or raises.
How do these variations affect your grow?
When taking pH level readings, many people take a solution into a controlled environment to get an accurate measurement of their solution. While this may seem like a good idea, the temperature of the fluid may be altered while in transit, thus changing the pH level. The improper reading may lead one to over or undercompensate for a change in pH, which may have devastating results on your crops.
What you should be doing!
We recommend taking your pH level tests in the same environment that the plants are in. If possible, right inside the buckets themselves. Should you prefer to take the pH outside of the bucket, extract the needed water into a container and immediately begin your pH testing. These processes will result in the most accurate readings of your water's pH value.
Article for reference and data:
https://www.westlab.com/blog/2017/11/15/how-does-temperature-affect-ph