
Receive Free Shipping On All Systems + Orders
Plants require 18 essential elements. Three of the basic elements are obtained by the plant from either water or carbon dioxide in the air [Carbon (C), Oxygen (O) and Hydrogen (H)]. Additionally, the plant derives from the roots three primary/macro nutrients – meaning they are needed in large quantities – [Nitrogen (N), Phosphorus (P) and Potassium (K)] as well as three secondary nutrients [Magnesium (Mg), Calcium (Ca) and Sulfur (S). The final 9 nutrients are considered essential micronutrients, and these too are derived from the plant's roots. "Essential micronutrient" means the plant requires micro-doses of these 9 nutrients to complete some aspect of its lifecycle. Deficiencies in essential micronutrients can result in problems with respect to plant growth, development and reproduction. The final 9 essential micronutrients are: Boron (B), Chlorine (Cl), Copper (Cu), Iron (Fe), Molybdenum (Mo), Manganese (Mn), Nickel (Ni), Zinc (Zn) and Silicon (Si). Each micronutrient plays an important role in the plant lifecycle even though the required dose can often fall below 10 ppm concentration in a deep-water culture system.
Of particular interest in this Tech Brief is Silicon, often described as elemental Silica or Silicon-Dioxide (SiO2). Silica is an intermediate uptake nutrient, meaning it is taken up by the plant through the roots at about the same rate as water uptake through evapotranspiration. In nutrient solutions, silica is typically derived from Potassium Silicate (K₂O₃Si) or Metasilicic acid (H2O3Si) which disassociates in water thus liberating silicon to be up taken by plants at reasonably high levels (depending upon plant needs). Silicon performs several important functions within the Cannabis sativa lifecycle:
So, we now have a sense of the importance of silica as an essential micronutrient. Whether it is a macronutrient, secondary nutrient or an essential micronutrient, it is important to track plant consumption of the nutrient mix being offered to plants growing in recirculating deep-water culture systems. At a minimum, keeping track of EC or electrical conductivity is critical in understanding plant health and harvest success. Unlike substrate methods wherein fertigation runoff (a requirement to assure the nutrient depleted root-mass is overwatered to replenish nutrients with a subsequent dry-back), recirculating deep-water culture always presents the root-mass exactly the necessary nutrient concentration without the 10-20% leachate runoff/waste of substrate fertigation. In fact, one can only surmise the nutrient concentration in substrate/fertigation farming techniques. In recirculating deep-water culture, the cultivator has the opportunity to evaluate nutrient concentrations and consumptions across the 15 root-delivered nutrients. All that is necessary, beyond utilizing Hydra Unlimited's recirculating deep-water culture system, is access to an A2LA accredited lab capable of evaluating nutrient water [internet keyword search: water quality lab].
of EC or electrical conductivity is critical in understanding plant health and harvest success. Unlike substrate methods wherein fertigation runoff (a requirement to assure the nutrient depleted root-mass is overwatered to replenish nutrients with a subsequent dry-back), recirculating deep-water culture always presents the root-mass exactly the necessary nutrient concentration without the 10-20% leachate runoff/waste of substrate fertigation. In fact, one can only surmise the nutrient concentration in substrate/fertigation farming techniques. In recirculating deep-water culture, the cultivator has the opportunity to evaluate nutrient concentrations and consumptions across the 15 root-delivered nutrients. All that is necessary, beyond utilizing Hydra Unlimited's recirculating deep-water culture system, is access to an A2LA accredited lab capable of evaluating nutrient water [internet keyword search: water quality lab].
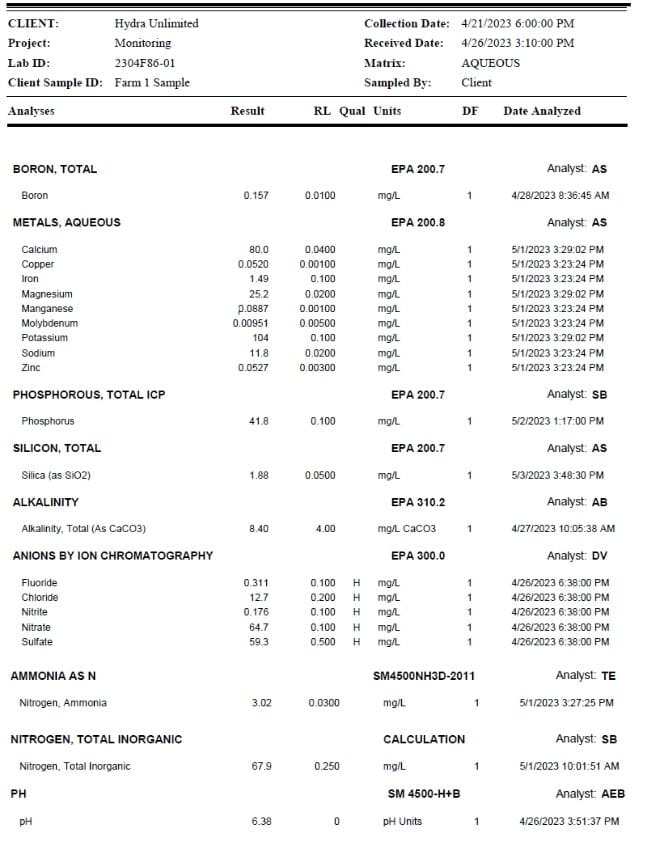
In the lab report shown here, we see a complete assessment of the recirculating nutrient water. Our NP- K ratio is listed as 1.65-1-2.5. We also see calcium at 80 ppm and magnesium at 25 ppm. Note too, that silica is at 1.88 ppm. We'll have to run some math to break out Si from the reported silica value (0.877 ppm silicon). [1 mg/l = 1 ppm]
Total cost for this assessment: $150 USD. This in-depth report of exactly what the roots are feeding upon is only available from samples taken from recirculating deep-water culture hydroponics systems such as offered by Hydra Unlimited. It is important to remember different facilities, different cultivars and different growing conditions will affect evapotranspiration rates and corresponding nutrient uptakes. We recommend our customers periodically run water sample assessments to baseline their nutrient plans and adjust based upon plant expression as well as chemistry/nutrient goals.
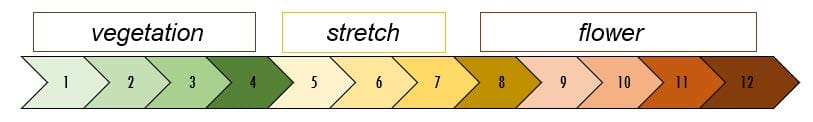
Armed with our water sample report and the knowledge that silica is an important "essential micronutrient" let's have some fun. We will investigate silica uptake during the growth phase commonly known as "stretch." If we break down the typical growth phases of Cannabis sativa into three parts over a 12-week period, we might see something like this: Vegetation, Stretch and Flower.

In more scientific terms, the "stretch" phase of a developing stem of Cannabis sativa contains a specific growth region called the 'snap point' where the fiber-enriched bast tissues considerably change their mechanical properties. Bast tissues are tissues along the stem axis of the Cannabis sativa which make up part of the phloem fibers. The "snap point" is present during a specific period of plant development—the fast growth phase, where elongation and secondary wall thickening of the stem occurs. The snap point disappears when stem growth is completed and the cultivars transition into the flower phase.
Our hypothesis: we hope to see biomineralization in the form of silica uptake during this stretch/snap point growth phase. We will investigate silica uptake spanning from week 4 through week 8 and expect acceleration of silica uptake at the start of the snap point with a slowing of silica uptake at the close of the snap point. We will perform weekly nutrient water sampling taken from 4 parallel systems occupying the same grow room. We will attempt to overlay the plant growth index (canopy volume) with measured silica concentration in the nutrient water.
[Growth Index: GI= π · (average canopy width/2)2 · height]
Where final plant height (from the soil level to the top point of the plant) is multiplied by "average canopy width," the average of the width measured at the widest point of the plant and 90° to the widest point. This generally calculates a plant canopy cylinder volume.
For further information regarding the "Snap point" – The snap point: A transition point in Linumusitatissimum bast fiber development [Gorshkova et.al ] November 2003, Industrial Crops and Products 18(3):213–221
The Boundary Conditions – How the experiment is set up

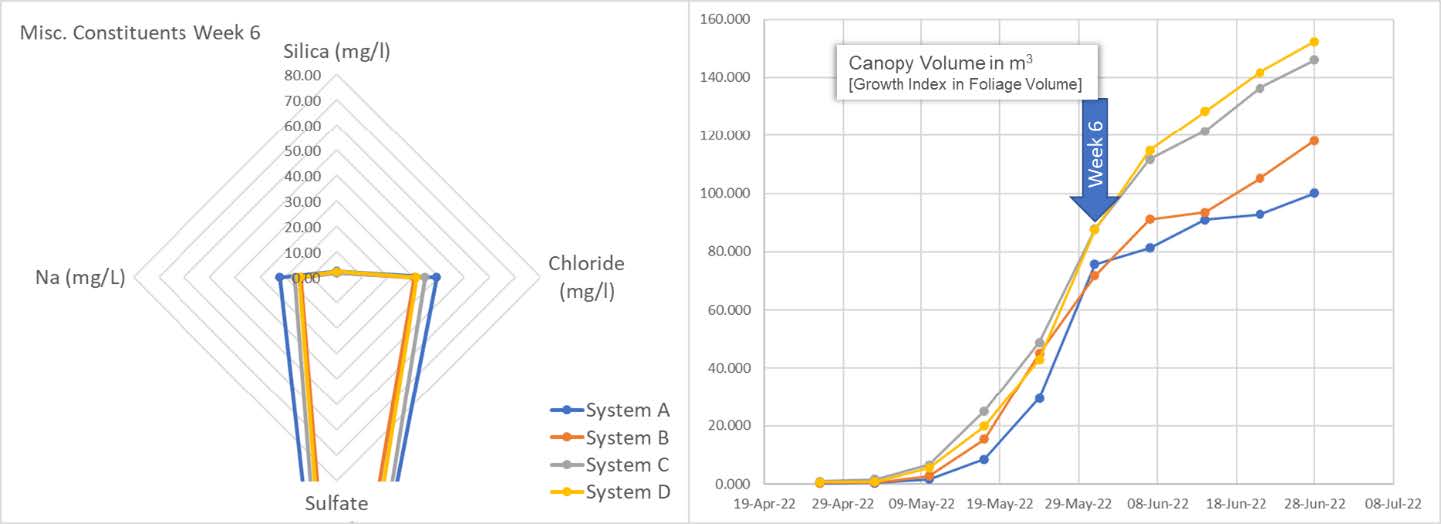
Here we see all 4 systems with a spider chart (left) showing not only silica (SiO2) concentration in mg/l (ppm) but also sodium (Na), chloride (Cl) and Sulfate (SO4). Silica is sitting at 25 ppm for systems B, C, D and at about 40 ppm for System A.
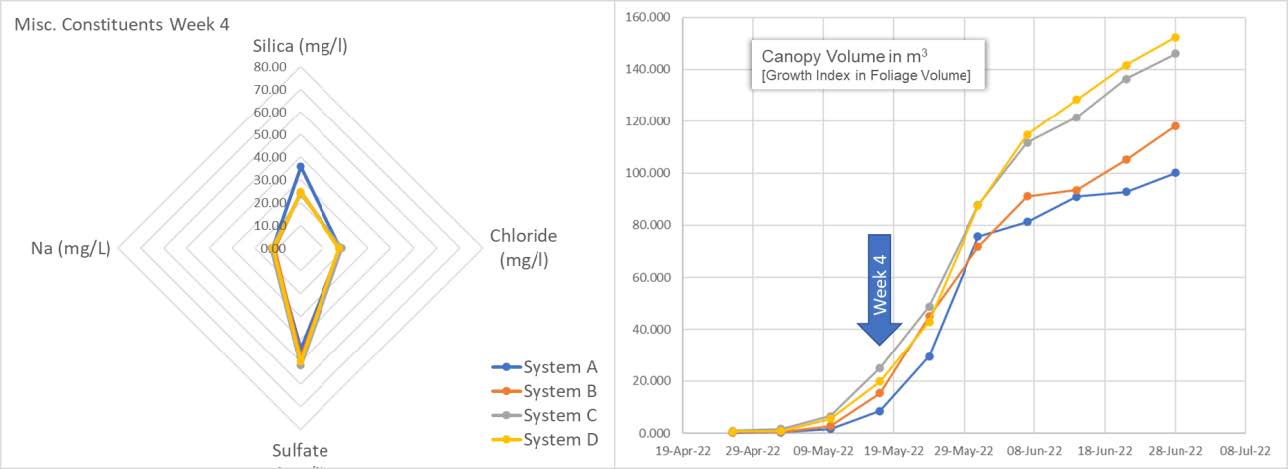
The graph on the right shows plant progress in canopy volume as calculated by the growth index. This is the average canopy volume per system.

The spider chart shows replenishment of systems B, C and D. We see the results of system A, being left as control, entering the snap point wherein A is beginning to consume residual Silica. We can also see from the graph on the left an acceleration of the growth index aligning with snap point "plant-stretch" progress.
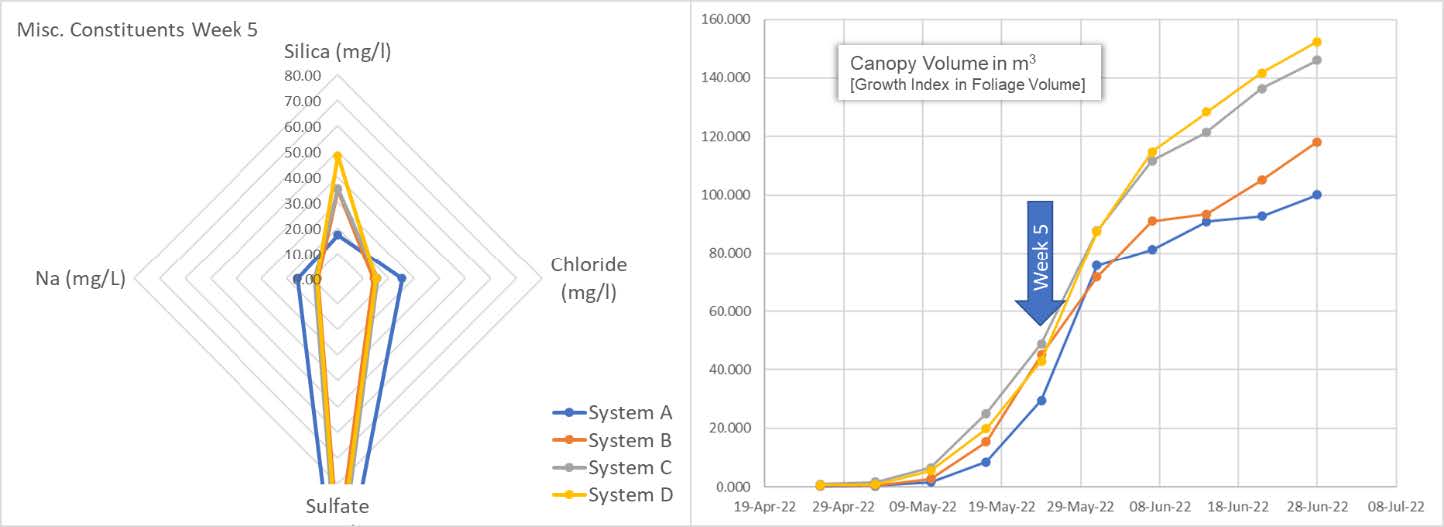

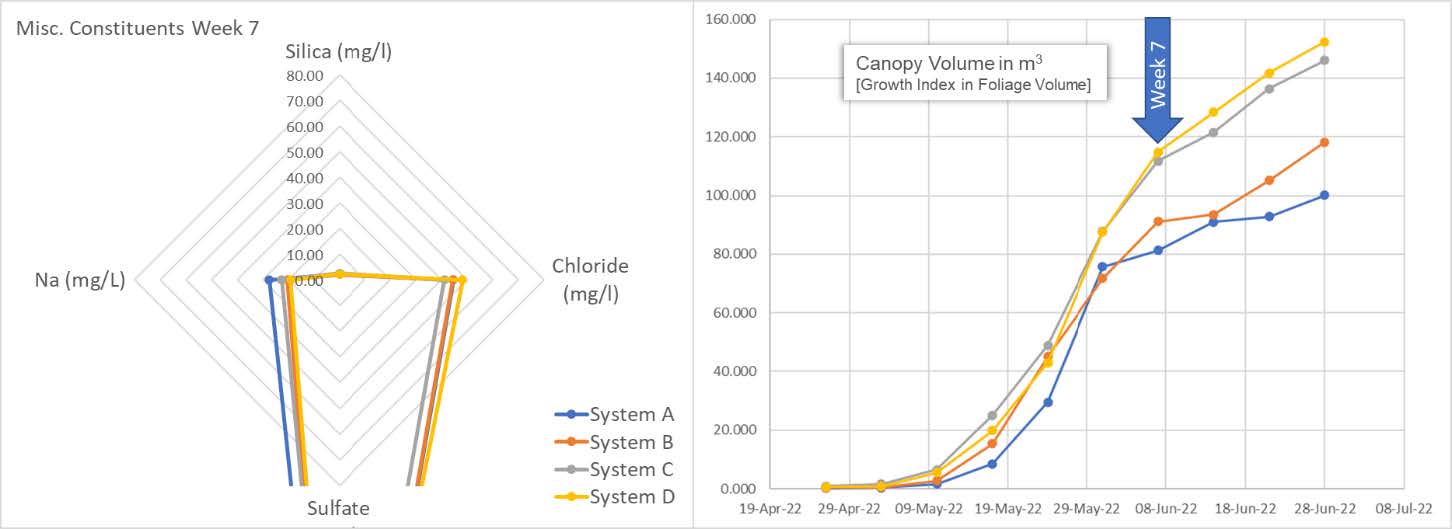
The spider graph reveals the plants are eating every bit of silica they can. The system is now depleted of this important micronutrient. On the right, we can see the snap-point is fully accelerating in terms of the calculated canopy volume. We also see an accumulation of Na and Cl in the system. It is unclear as to the increasing source of these elements and begs further study.
Here we also get a sense of the difference between below average and above average clones. System A and B cultivars begin to show their lack of vitality as compared to the cultivars in system C and D. Independent of vitality, all are still consuming and depleting the available silica.

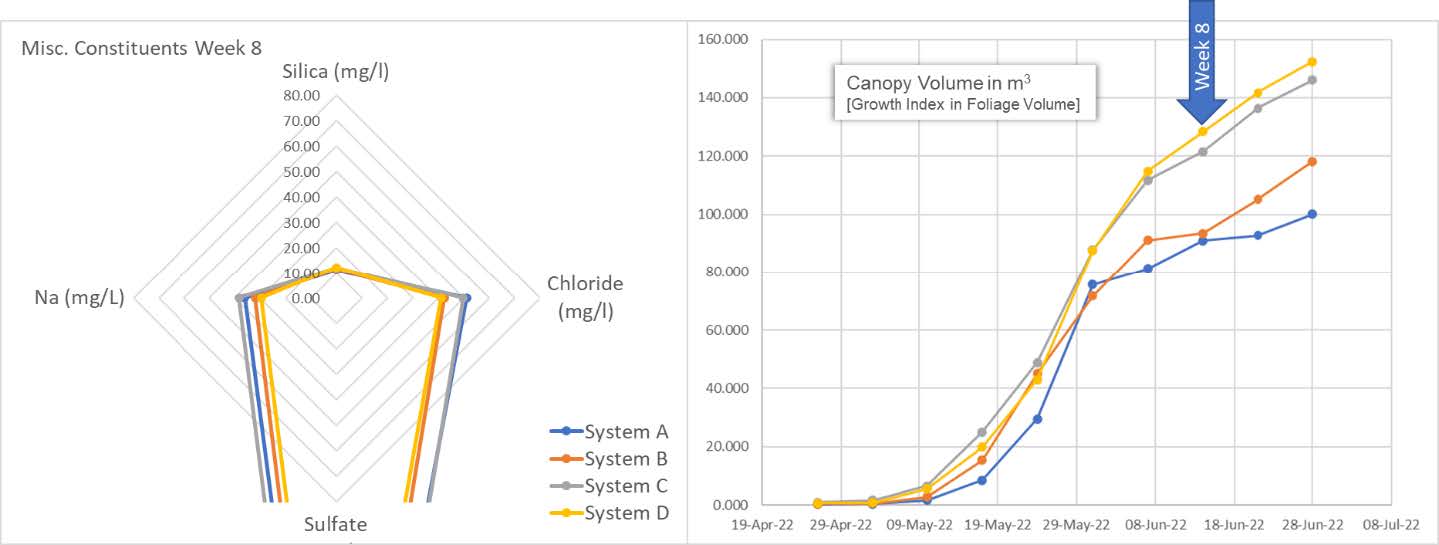
We begin to exit the snap point for the cultivars as we see a corresponding change in slope (rate of change of plant progress). And yet, the plants continue their voracious appetite for Silica. [Note: control system A; growth index measurement anomaly week 7 has under-represented actual growth index. Corrected for the following week].
The entire story is told by the spider graph on the left. The plants in all four systems end their massive uptake of silica and exit the snap point. As stated earlier: The "snap point" is present during a specific period of plant development—the fast growth phase, where elongation and secondary wall thickening of the stem occurs. The snap point disappears when stem growth is completed.
The hypothesis stated earlier cannot be fully validated without tissue samples to verify silica translocation to the bast fibers for each week of the snap point. And while we will leave the tissue assessment for further study, what can be learned is the incredible power of Hydra Unlimited's recirculating deep-water culture system for nutrient assessment. No other cultivation technique affords this level of insight into what the plants are actually eating as they progress through their life cycle.
We can take the recognition of silica depletion to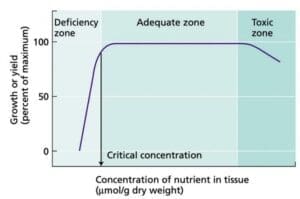 the next step. The graph to the right depicts resulting growth success as a function of nutrient concentration. Too few or too much of a particular nutrient can adversely affect plant success. In our silica example, a case could be made where silica has fallen to the deficiency zone. Remediation steps of offering replacement silica to the water in the form of potassium silicate or metasilicic acid could be considered in order to replenish and return silica concentrations back into the cultivator's planned "adequate zone" for silica concentration.
the next step. The graph to the right depicts resulting growth success as a function of nutrient concentration. Too few or too much of a particular nutrient can adversely affect plant success. In our silica example, a case could be made where silica has fallen to the deficiency zone. Remediation steps of offering replacement silica to the water in the form of potassium silicate or metasilicic acid could be considered in order to replenish and return silica concentrations back into the cultivator's planned "adequate zone" for silica concentration.
In this tech brief we investigated silica uptake. Macro nutrients can also be studied to this level of detail. Others? Calcium and Magnesium next? Maybe Boron?
The methods and techniques shown here can also be applied to the next level of crop steering – beyond manipulating the environment. We have the potential to tailor our nutrient choices to modulate the expression of phenotype and potentially influence genotype as well as chemotype expression. Through the use of Hydra Unlimited's recirculating deep-water culture system paired, with water chemistry assessment and data visualization, it's possible to bring crop steering to the next level.
H. W. (Hank) Bonnah | Chief Scientist / Principal Engineer
Contact Hydra Unlimited, 960 74th St. SW, Byron Center, MI 49315.
Made in the USA, Hydra Unlimited's HydraMax is a complete commercial deep water culture system. With best-in-class aeration, nutrients are quickly and evenly delivered to each bucket simultaneously. This results in consistently faster vegging, higher yields and greater profits.